During normal physiological wound healing, inflammation, angiogenesis and fibrogenesis are all effectively controlled, so that once sufficient matrix has accumulated in the wound bed, matrix production ends and scarring is prevented. Should this response fail to resolve, or the tissue continues to receive injury, chronic fibrosis and scar tissue formation can occur.
Transforming growth factor beta (TGF-β) levels regulate several biological processes including cell growth and differentiation, apoptosis, cell migration, immune cell function and extracellular matrix (ECM) production. TGF-ß1 signalling occurs principally via SMAD-2/3, which are part of the receptor-activated SMAD (R-SMAD) family. TGF-ß activates cell-surface receptors, which then phosphorylates SMAD-2/3 proteins residing in the cytoplasm. These phosphorylated SMAD complexes translocate to the nucleus, where they regulate gene expression. Phosphorylation and dephosphorylation of SMADs is a key indicator of target engagement in therapeutic interventions.
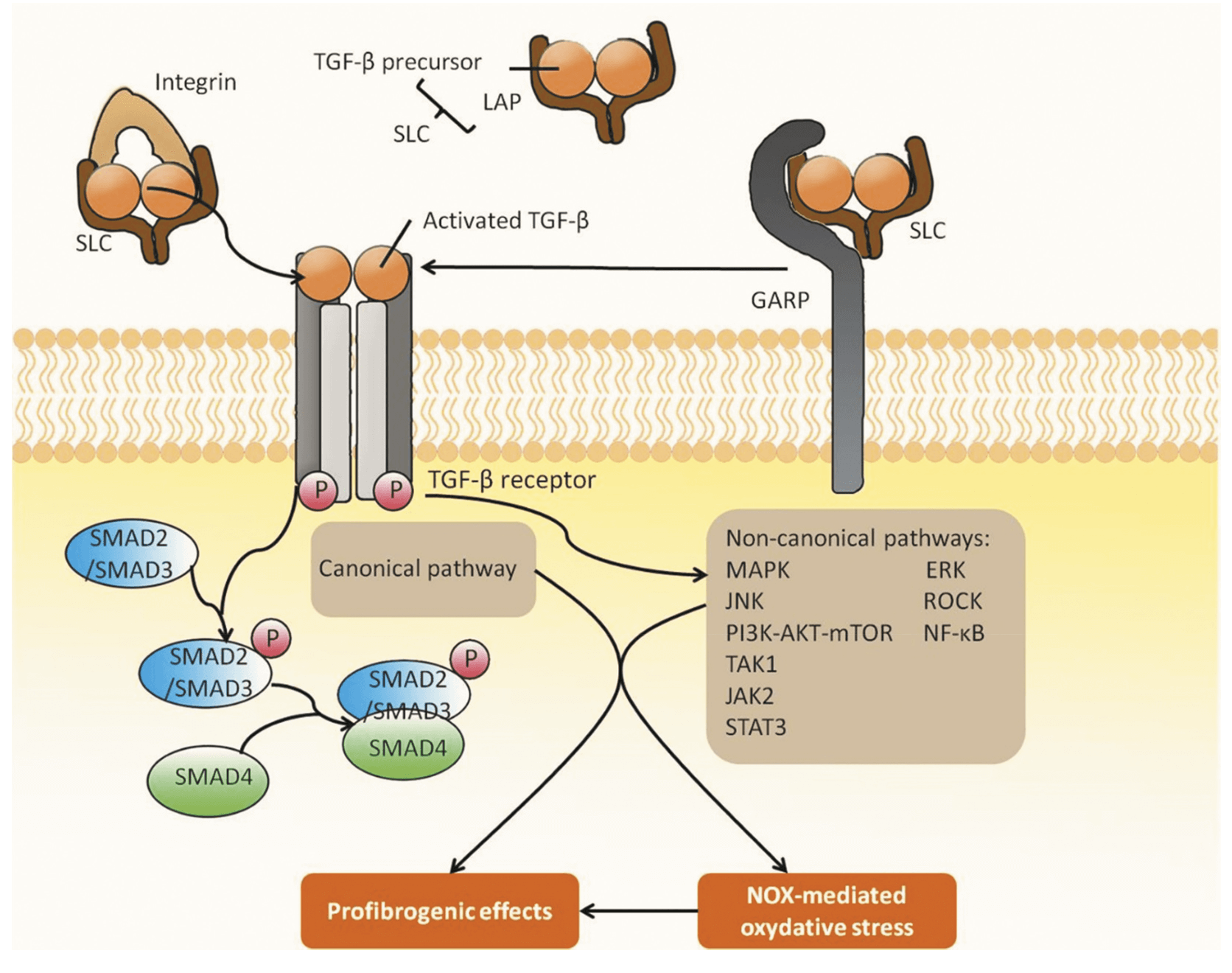 TGF signalling in fibrosis. Reference: Li et al. 2021. Expert Opin. Ther. Pat. 10.1080/13543776.2021.1896705
TGF signalling in fibrosis. Reference: Li et al. 2021. Expert Opin. Ther. Pat. 10.1080/13543776.2021.1896705
TGF-ß levels can contribute to fibrosis not only through the accumulation of ECM but also through downregulation of matrix-degrading enzymes and increased expression of MMP inhibitors. This latter process is also mediated via SMAD3 signalling. TGF-ß1 has a natural affinity for ECM and concentrates in the fibrotic site, thus exacerbating the condition. Fibrosis can affect multiple organs including the skin, liver, kidney, eye, lungs and heart, all of which can be associated with chronic fibrotic diseases.
Management of TGF-β-mediated fibrosis has led to the development of different therapeutics with several targets such as integrins, which mediate downstream TGF-β through binding to the TGF-β receptor. One third of integrins bind directly to the ECM, therefore small molecule, peptide and antibody therapies have been developed to target this binding via the integrin receptors or integrins directly. Other therapeutic targets include Glycoprotein-a repetition predominant protein (GARP), Matrix metalloproteinases (MMP), both of which contribute to TGF-β activation. TGF-β monoclonal antibodies, ligand traps and TGF-β kinase inhibitors are further examples of targets. As SMADs are part of the main canonical pathway for TGF-β signalling, they are also promising targets to reduce fibrosis.
Decoy oligonucleotides which targets SMAD, inhibit the transcription of TGF-β1 and SMAD genes. Small molecules and recombinant fusion proteins mediate SMAD negative feedback, further reducing TGF-β signalling.
At Synexa Life Sciences, we have established methods for the quantification of SMAD2/3 in bronchiolar lavage cell lysate, stimulated peripheral blood mononuclear cells (PBMCs) and hepG2 cells. SMAD phosphorylation remains a key biomarker in fibrosis as well as assessing clinical efficacy of anti-fibrotic treatments. More info on our SMAD assays can be found here.