Pre-eclampsia (PE) is a condition that affects up to 8% of pregnancies worldwide. The disease has a multi-organ impact and contributes significantly to the burden of maternal morbidity. In the developing child, PE often causes growth restriction and alters oxygenation and nutrient transfer, which in many cases precipitates pre-term delivery. Currently, delivery of the neonate is the only available treatment for severe PE. Low-dose aspirin and bed rest can be used to prolong pregnancy in some cases, depending on the point of gestation where PE diagnosis occurs.
PE diagnosis includes new onset hypertension and raised urinary protein levels and can present with and without severe factors (Figure 1).
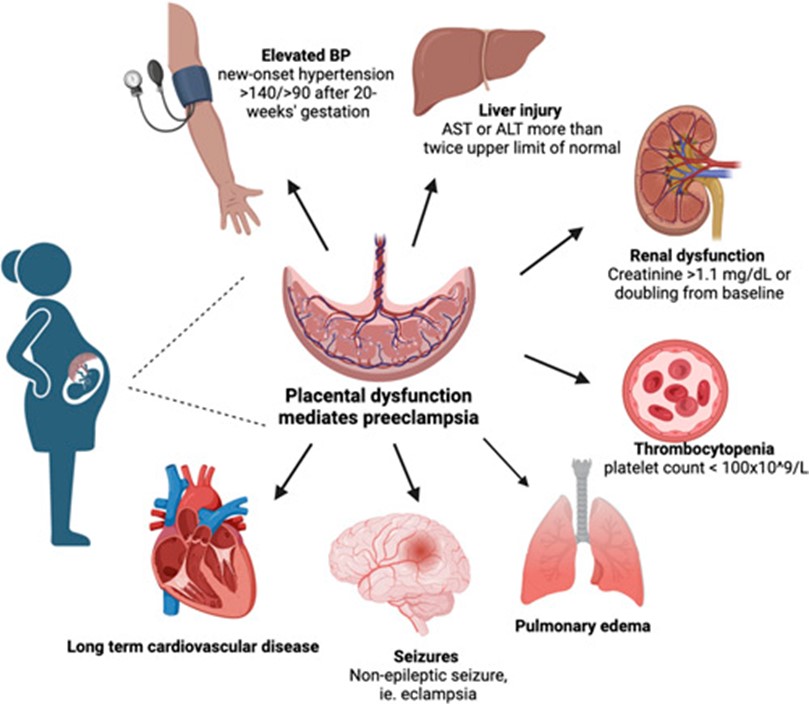
Early onset PE is characterised as PE occurring before the 34th week of pregnancy. Although early onset is less common, occurring in 5-20% of PE cases, it is associated with a heightened risk of HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome. HELLP is a severe PE complication which predisposes mothers to eclampsia, new onset grand mal seizure and/or coma.
Pathophysiology
The pathophysiology of PE is ill-defined, particularly in relation to the development of HELLP. The first stage involves abnormal placentation, deficient trophoblast invasion and insufficient remodelling of uterine spiral arteries, leading to reduced placental perfusion. These combined factors cause the release of antiangiogenic factors. The second stage includes changes in maternal vascular function, vascular narrowing, oedema and platelet loss. Endothelial cell disruption is thought to cause an increase in liver enzymes, increasing prostacyclin and thromboxane, causing hepatic vessel constriction, hepatic hypoxia, necrosis and hepatocyte degeneration. HELLP syndrome is associated with a rare but life-threatening complication: subcapsular hematoma.
The need for early detection biomarkers
Atypical cases of PE where pregnant women don’t present with both hypertension and elevated urinary protein are relatively common, particularly in those with pre-existing conditions. Critically, there is a lack of assays highly specific for PE. The search for a useful biomarker for high-risk cases is crucial. There exists a time-dependent and highly consequential balance between symptom management and early-term delivery. Delivery too early exposes the neonate to premature complications while a delivery too late could result in both mother and child exposed to an elevated risk of severe manifestations.
The most currently utilised biomarkers include assays to quantify placental growth factor (PIGF) and soluble FMS-like tyrosine kinase 1 (sFlt1). PIGF promotes new blood vessel formation, while sFlt1 inhibits the activity of PIGF. Therefore the sFlt1:PIGF ratio is informative in determining the risk of pre-term PE. This ratio, however, has a relatively low predictive value. Studies have yet to demonstrate that pregnancy outcomes are improved by screening cases using this ratio.
The application of extracellular microRNA’s (ex-miRNA)
A recent study used an unbiased transcriptomic approach to examine ex-miRNAs for early PE diagnosis and for post hoc analysis for severity prediction. RNA was extracted from maternal serum samples and then subjected to downstream RNA-seq. The ex-miRNAs were ranked according to their predictive value in the discovery cohort and then evaluated in the verification cohort. The authors identified candidate univariate biomarkers which exhibited strong separation between case and controls. Most of the identified 10 candidate univariate ex-miRNAs could further distinguish cases on disease severity. These ex-miRNAs were predicted to originate from the liver, placenta, platelets or red blood cells.
The authors further detailed a list of 110 bivariate paired ex-miRNA biomarkers. This assay/approach performed similarly to the FDA-approved sFlt1:PIGF ratio. Further, applying bivariate ex-miRNA and the sFlt1:PIGF ratio sequentially improved both sensitivity and specificity for early PE diagnosis. Further validation studies will be required to establish the clinical utility of this approach fully. Once validated, these biomarkers, when used in patient screening, will allow for better resource allocation and decrease the need for low-risk patients to be exposed to unnecessary risks and procedures. Importantly, additional studies will help increase the understanding of the roles these ex-miRNA play in disease pathology, which may help in the development of highly PE-specific therapies.
Closing remarks
PE remains a significant and highly destructive disease with very limited options. Early detection remains one of the few open avenues to alleviate disease burden, until a more effective, targeted therapy becomes available. The data on ex-miRNA is particularly promising as it has identified a myriad of potential pathophysiological pathways worth investigating. These efforts all help the global understanding of disease progression and the power of unbiased biomarker discovery.
About Synexa and Scientific Strategies
Synexa is a specialised biomarker and bioanalytical services firm, established over 20 years ago. We support customers at all stages of clinical development, from discovery to phase 3 studies. Our services cover analysis across the biological continuum, from nucleic acid, soluble biomarkers, cell and tissue. Our team of dedicated scientists, working across labs in South Africa, Europe, the UK and the US, have supported clinical programs in many therapeutic areas and our experience continues to grow. We continue to innovate, broadening our service offering with cutting-edge technology platforms which are instrumental in discovering and measuring disease-associated biomarkers.
A component of our broader service offering is Synexa’s Scientific Strategies Team. There are many challenges and risks in progressing a compound into clinical development; the Scientific Strategies team can help de-risk your development by providing support around key biomarker and bioanalytical considerations. The dedicated team can offer standalone consulting support, providing objective, actionable solutions to your biomarker and bioanalytical challenges.
For more information, get in touch with us at contactus@synexagroup.com.
References:
- Mei and Afshar. Clin Liver Dis. 2023 Dec 8;22(6):195-199
- Morey et al. Sci Adv. 2023 Dec 22;9(51):eadg7545.





