“Prion-like” misfolding, aggregation and accumulation of host proteins are key pathological drivers of neurodegenerative diseases. Examples of these proteins include β-amyloid, tau, TAR DNA-binding protein 43 (TDP-43) and α-synuclein (1). The latter has garnered increased attention in 2023, largely due to the increased urgency in identifying prognostic biomarkers of Parkinson’s disease (PD).
The pathology of PD is multifactorial and can be linked to both genetic and environmental factors, however, there has still been no direct cause clearly defined. It has been difficult to link specific biomarkers to the onset of disease, therefore, the announcement of a potentially prognostic biomarker of PD in early 2023 was incredibly exciting.
Aggregation of α-syn, due to misfolding, causes accumulation in neural cells to toxic levels, resulting in neuronal cell death, playing a central role in the development of PD. Alpha-synuclein can exist in multiple forms with increases in oligomeric α-syn pathologically implicated in the progression of PD. Direct measurement of oligomeric α-syn has been suggested as a possible prognostic biomarker, however, reliable and consistent assays have yet to be developed.
Seed amplification assays have been widely applied with great success in prion disease research and are used to detect the presence of proteopathic seeds, which are self-propagating misfolded host protein assemblies which can seed the misfolding of normal host proteins (1). This assay principle has now been applied to studying neurodegenerative disease.
To detect low concentrations of amyloid fibrils, seed amplification assays (SAA) have been developed. For the remainder of this article, we will focus on the methodology of these assays, the current status of their clinical use and the next steps for standardization.
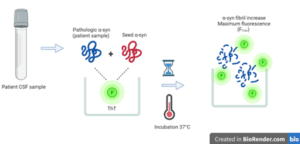
Figure 1. Simplified SAA workflow. Cerebrospinal fluid (CSF) patient samples are incubated along with seed alpha-synuclein (α-syn) and fluorescent dye, ThT in microwell plates at 37°C with shaking to induce amplification and an increase in fluorescence up to Fmax.
SAA’s are not complex assays to run, demonstrate high specificity and sensitivity. The basic principle involves adding non-aggregated monomeric α-syn to the sample of interest. The assay has been applied to serum and skin, but is currently, most commonly used with cerebrospinal fluid (CSF) samples. Pathologic seeds in the sample will recruit monomeric α-syn to elongate into longer fibrils. By shaking the sample, the fibrils fragment, increasing their number. The newly created fibrils continue to recruit more monomeric α-syn and the cycle continues. This amplification is monitored with a fluorescent dye (thioflavin T, ThT) which binds amyloid (2). When bound to amyloid, ThT becomes strongly fluorescent, allowing total fluorescence to serve as indication of seed amplification. In their current application SAAs, are used to determine whether seeds are present or not and is therefore empirically qualitative.
Although not technically complex, SAA’s can take long to perform. A method published in Nature Protocols detailed an SAA method in which the reaction is carried out for up to 17 days (3). Further assay optimizations have reduced this time but conducting the assay remains as a significant drain on resources in bioanalytical labs. The aforementioned method remained qualitative, relying on results from three microplate wells to distinguish: α-syn-SAA positive (all three wells positive), inconclusive (two wells positive) or negative (one or no wells positive). Positivity is assessed by comparing maximum fluorescence (Fmax) to the fluorescence threshold of the instrument. If Fmax > threshold, the sample is positive (3).
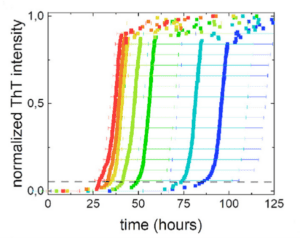
Figure 2. ThT fluorescence intensity over time with different seed concentrations. Red = high concentration and blue = low concentration. From Vaneyck et al. J. Phys. Chem. B. 2023. 127(8): 1735-1743
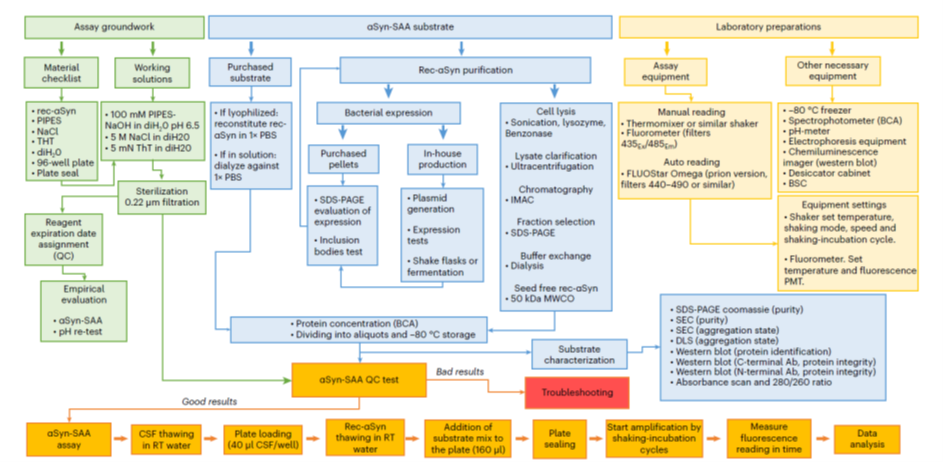
Figure 3. SAA workflow. From Concha-Marambio et al. Nature Protocols. 2023. 18: 1179-1196. Blue boxes pertain to substrate production and characterization.
Attempts to make the SAA quantitative have included using end-point dilution assays or digital microfluidics. A study published in February 2023, provided another option, which quantifies time required to reach ThT fluorescent threshold (2). The assay relies on using standard amount of synuclein seeds. The authors found a clear relationship between seed concentration and fluorescence intensity over time (Figure 2). They performed several robustness experiments, including differences in salt conditions and matrix types to gauge interference and determine mitigation.
In conclusion, this study provides a promising and important step in using SAA’s as quantitative tests. While used in clinical trials, SAA’s are often used in qualitative screening for inclusion in PD studies. The SAA is a powerful test, which has already shown usefulness in early PD prognosis, as published in the Lancet (4). Its use as a quantitative clinical marker still has some significant challenges to overcome. The most principle of which, is standardizing the approach.
This includes:
- Fluorescence parameters and instrumentation to keep fluorescence threshold consistent between analytical labs.
- Seed controls:
- In published studies and protocols using the SAA, seed synuclein is often recombinantly produced, requiring a highly controlled and validated workflow of its own (Figure 3) .
- There are commercial options, however, these are bespoke client requests and are not widely available.
- For better harmonization, an international standard with a known validated seed concentration would be the most ideal.
- Assay time:
- It is important to maximise efficiencies and shorten assay incubation times to avoid analytical bottlenecks, particularly in larger trials.
- SAA’s are only effective when de novo fibril formation is slow or inefficient compared to fibril amplification. There is therefore significant development required in each assay to ensure clinical samples can be accurately measured with the available assay.
Blog written by Dr. Nicholas Woudberg (PhD), Head of Scientific Strategies
References
- Coysh T, Mead S. The Future of Seed Amplification Assays and Clinical Trials. Front Aging Neurosci [Internet]. 2022 [cited 2023 Aug 25];14. Available from: https://www.frontiersin.org/articles/10.3389/fnagi.2022.872629
- Vaneyck J, Yousif TA, Segers-Nolten I, Blum C, Claessens MMAE. Quantitative Seed Amplification Assay: A Proof-of-Principle Study. J Phys Chem B. 2023 Mar 2;127(8):1735–43.
- Concha-Marambio L, Pritzkow S, Shahnawaz M, Farris CM, Soto C. Seed amplification assay for the detection of pathologic alpha-synuclein aggregates in cerebrospinal fluid. Nat Protoc. 2023 Apr;18(4):1179–96.
- Siderowf A, Concha-Marambio L, Lafontant DE, Farris CM, Ma Y, Urenia PA, et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 2023 May 1;22(5):407–17.




