In order to combat disease, host organisms have an array of defence mechanisms, some of which have been the foundation for groundbreaking discoveries. Famously, the way bacteria fight infection helped us develop the revolutionary CRISPR gene editing technology.
Pattern recognition receptors detect the presence of microbial replication and trigger downstream inflammatory pathways to combat infection. Examples of these receptors include the toll-like receptors, NOD-like receptors and cGAS (cyclic GMP-AMP synthase). cGAS binds to and detects double-stranded DNA and following this binding, releases cGAMP, which in turn binds to and activates STING. Through a series of additional steps, STING triggers the release of interferon-1-related pro-inflammatory cytokines. The inherent issue with cGAS though, is that the DNA binding is not sequence-specific, meaning this protective mechanism can in fact turn on its host.
The cGAS/STING pathway
Chronic STING activation is a hallmark of autoimmune conditions including lupus, Aicardi–Goutières syndrome, STING-associated vasculopathy with onset in infancy (SAVI) and continues to be linked with more and more disease conditions. In this latest blog, we explore this pathway, its burgeoning links to multiple diseases and how biomarker profiling can assist in the development of targeted and effective therapies.
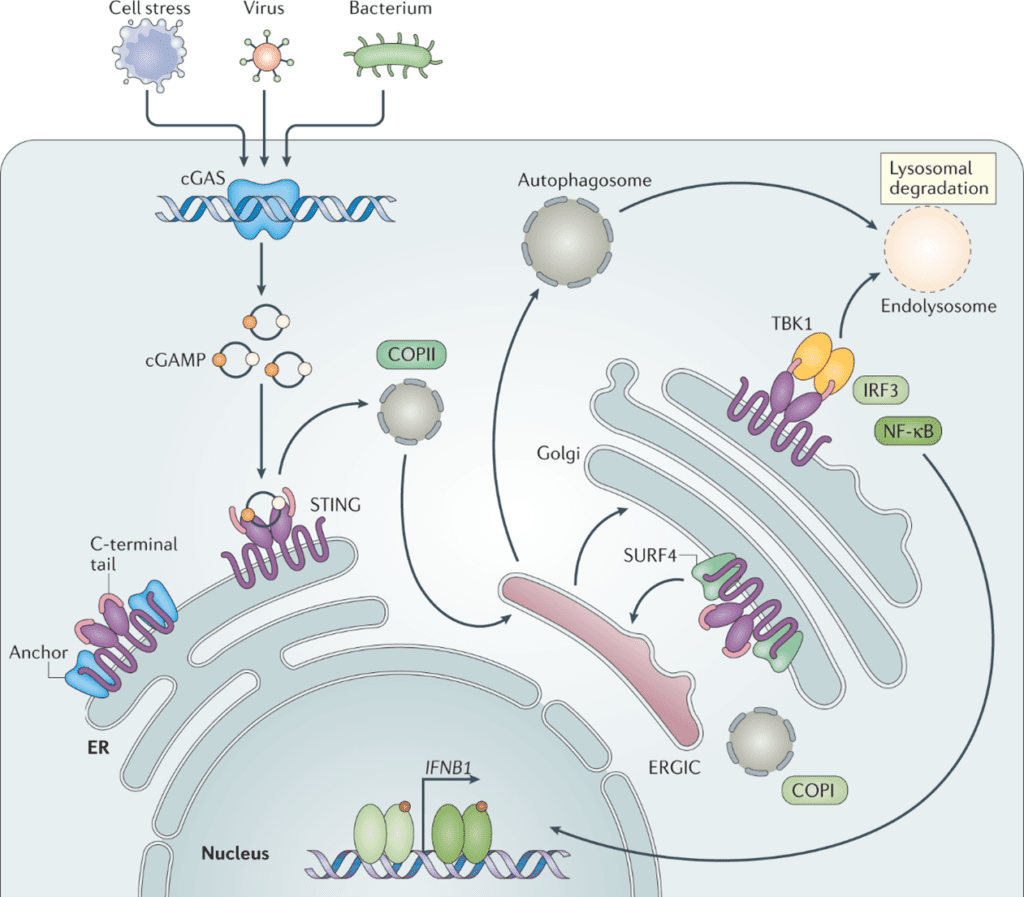
Figure 1. The cGAS/STING pathway, from Decout et al. Nat Rev Immunol 21, 548–569 (2021).
dsDNA binding to cGAS activates it, which releases cGAMP. cGAMP is detected by STING in the ER membrane. Once activated by cGAMP, STING translocates to the Golgi, recruits TBK1 to initiate phosphorylation at Ser366. Phosphorylated STING (pSTING) can then recruit IRF3, inducing the transcription of interferon-associated genes and release of inflammatory cytokines. Ordinarily, these inflammatory cytokines and interferon-associated proteins assist with the immune response to infection, however, when chronically or erroneously activated, is linked to many of the hallmarks and pathology of diseases.

Figure 2. Involvement of cGAS and/or STING in disease. From Skopelja-Gardner, An & Elkon. Nat Rev Nephrol 18, 558–572 (2022).
cGAS/STING contribution to disease pathology
As mentioned before, chronically elevated levels of type 1 interferons are observed in systemic autoimmune conditions including lupus, Sjögren syndrome, scleroderma and dermatomyositis. Whilst also attributed to the TLR’s, the cGAS/STING pathway has been heavily implicated in these conditions and are the target of several clinical programs.
Beyond autoimmune disease, the cGAS/STING pathway has been associated with several other disease indications (summarised in figure 2).
One of the primary drivers of cGAS activation in several disease conditions is mitochondrial DNA (mtDNA). Mitochondria, under conditions of stress and dysfunction, can leak and release mtDNA into the cytoplasm where these can interact with and activate cGAS. mtDNA-linked activation of cGAS has been implicated in multiple diseases including:
- Chronic kidney disease
- Lung fibrotic diseases
- Neurodegenerative conditions such as Parkinson’s disease
In Parkinson’s disease specifically, a lack of protective proteins make mitochondria more susceptible to damage and oxidation. From a clinical perspective, measurement of mtDNA and mitochondrial retention could be key biomarkers in understanding disease progression, risk and response to potential therapies which mediate the cGAS/STING pathway.
The association between cGAS and cardiac diseases has gained significant attention recently with inhibition of STING-induced inflammation showing benefit in multiple cardiovascular diseases including atherosclerosis, heart failure, myocardial infarction and myocarditis. In the latter case, cGAS inhibition was shown to reduce the risk of immune-checkpoint inhibitor-induced myocarditis in a study published earlier this month: Sun et al. Nat Commun 15, 6640 (2024). Mechanistically, preclinical studies have shown that inhibition of cGAS not only reduces inflammation but also cardiac fibrosis, apoptosis, pyroptosis, whilst preserving myocardial function and improving plaque stability. Similarly to other diseases, it is thought that mitochondrial damage, cell death of immune, vascular endothelial and smooth muscle cells, common in cardiac disease pathology, leads to chronic cGAS/STING activation. Its rational to consider then; how cGAS and STING may be attractive targets in cardiovascular disease therapies, although due to inherent complexities in these diseases, further exploration is likely needed.
Beneficial cGAS/STING activation in cancer
Interestingly for this pathway, as much as there is active work in subduing it, there is a targeted effort in activating it. In the realm of cancer immunotherapy, STING activation and type 1 interferons are important for optimal tumour antigen-specific CD8+ T-cell priming. The question however is, how do tumor cells activate STING in immune cells? It has been proposed that tumour-derived cGAMP or tumour-derived DNA are transferred to dendritic cells. To avoid DNAses and lysosomal degradation, it is theorised that tumor DNA is packaged within exosomes prior to transfer to dendritic cells. This, however, remains an area requiring further study. What is clear, however, is that STING agonism, either alone or in combination with targeted anti-tumor therapy is a growing therapeutic modality in cancer.
Biomarker strategies for cGAS/STING candidates
At Synexa, we support our customers in defining bespoke biomarker strategies for early translational and clinical phase studies. The ultimate goal is combining sets of biomarkers which can both stratify patients ans also demonstrate the pharmacodynamic effects of a therapy. We have had the opportunity to establish assays which address key points in the cGAS/STING pathway and have explored these biomarkers in Translational Solutions studies. Using in vitro assays, we have been able to explore how cGAS/STING targeting therapies engage with their targets as well as define the biomarker landscape in target population groups.
If you would like to learn more about how Synexa’s services may support the journey of your clinical candidate before and in the clinic, please don’t hesitate to contact us.
References
- Decout, A., Katz, J.D., Venkatraman, S. et al. The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021. 21, 548–569
- Flood BA, Higgs EF, Li S, Luke JJ, Gajewski TF. STING pathway agonism as a cancer therapeutic. Immunol Rev. 2019 Jul;290(1):24-38.
- Kwak H, Lee E, Karki R. DNA sensors in metabolic and cardiovascular diseases: Molecular mechanisms and therapeutic prospects. Immunol Rev. 2024 Aug 19.
- Skopelja-Gardner, S., An, J. & Elkon, K.B. Role of the cGAS–STING pathway in systemic and organ-specific diseases. Nat Rev Nephrol. 2022. 18, 558–572
- Zhang Q, Shen L, Ruan H, Huang Z. cGAS-STING signaling in cardiovascular diseases. Front Immunol. 2024 May 13;15:1402817.
FAQ
How do you design biomarker strategies for cGAS–STING‑targeted programs where on‑target inflammation is a double‑edged sword?
Balance is key: panels should cover proximal pathway modulators (pSTING, IFN‑stimulated chemokines) alongside broader safety cytokines to detect overshoot. Synexa combines phospho‑protein workflows (targeted LC‑MS/MS phosphosite panels) with Olink interferon signatures and immune phenotyping for myeloid/lymphoid activation states. Time‑course sampling distinguishes transient desired activation from sustained harmful signalling. These data guide dose scheduling, combination strategies, and stopping rules.
How can translational models predict patient heterogeneity in STING responses?
Using fresh donor material, Synexa assesses inter‑individual variability in interferon tone, TLR cross‑talk, and myeloid priming to map response strata. High‑parameter flow plus cytokine kinetics reveal subgroups prone to hyper‑inflammation versus controlled activation. Linking these patterns to genetic or proteomic baselines enables pre‑emptive stratification in early trials. This reduces risk of idiosyncratic toxicity obscuring true efficacy.
What endpoints help differentiate STING agonists from antagonists in early development?
For agonists, look for rapid, dose‑responsive IFN‑I signature induction with controlled resolution and evidence of antigen‑presentation improvements; for antagonists, monitor suppression of those same axes without global immunosuppression. Synexa sets paired biomarker panels so directionality of change is unambiguous and quantifiable. Functional assays (antigen presentation, T‑cell priming) provide mechanistic corroboration. This clarity accelerates asset positioning and combination planning.