Today, 30th of May 2024, marks World Multiple Sclerosis (MS) Day. MS is a devastating autoimmune disease, characterised by neuronal inflammation which damages the myelin sheath, causing the demyelination of neurons. Demyelination disrupts neuronal signal transmission, which presents physically in patients as sensory disorder, motor dysfunction, optic neuritis and issues with coordination. Patients suffer through multiple relapses which in turn worsen the disease.
The pathophysiology of MS is linked to neuronal infiltration by autoreactive immune cells. It is thought that adhesion molecules, like integrins, play central roles in facilitating the migration of these cells from the periphery into the brain. Integrins are expressed on circulating lymphocytes which bind to adhesion molecules like VCAM, expressed on endothelial cells lining blood vessels around the brain (Figure 1A). Integrins therefore control the homing of autoreactive effector immune cells.
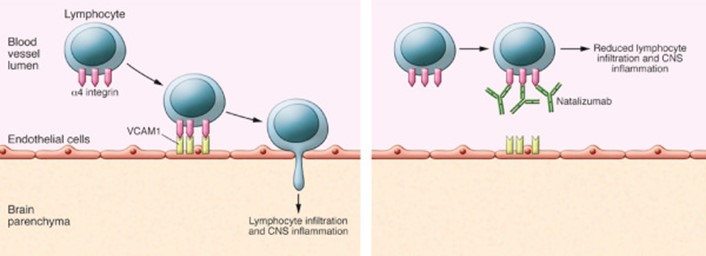
Integrins have consequently been a key therapeutic target in MS. Animal experiments demonstrated that blocking alpha-4-beta-1 (α4β1) prevented white blood cell infiltration into the brain in a mouse model of MS. A monoclonal antibody called Natalizumab, which binds to the α4 integrin, entered clinical trials in the late 90’s and early 2000’s (Figure 1B). The drug demonstrated effective reduction in active lesions, the number of relapses and increased the duration of relapse-free periods. As a result, Natalizumab was approved by the FDA for relapsed MS treatment in 2004. The therapy, however, was not without issues.
Shortly after approval, it was noted that a potentially fatal complication could result from treatment. An opportunistic infection from human polyomavirus 2 or John Cunningham (JC) virus can cause multifocal leukoencephalopathy (PML), an infection of the brain. Alpha4 integrin, the target of Natalizumab, in addition to controlling leukocyte homing, also has a role in maintaining anti-JC virus immunity. Due to the risk, the FDA withdrew approval for Natalizumab months after its initial approval. However, upon review and careful consideration of these risks, they re-instated the drug with anti-JC virus antibodies being a key predictive biomarker. Natalizumab remains the most potent treatment for relapsing MS and is currently prescribed in the US using the enhanced patient safety prescribing program which has additional means of monitoring patient safety.
It has been 20 years since the approval of the first monoclonal antibody treatment for relapsing MS, however, the drug remains expensive and largely inaccessible for many patients. In the pharmaceutical industry, generic compounds or in the case of biologic drugs like Natalizumab, biosimilars allow for a broader access to less expensive treatments which are deemed just as effective as the original drug. In January 2024, Sandoz launched Tyrunko® as the first and only biosimilar for MS treatment. To gain approval, the biosimilar drug needed to demonstrate high biological similarity to the originator compound as well as having similar safety, efficacy and biodistribution. These latter aspects are assessed in clinical trials where one patient group receives the new biosimilar drug and others receive the originator. Key characteristics of the drugs are compared including how they are processed in the body, the pharmacokinetics, and how the immune system responds, immunogenicity. The release of Tyrunko® is an important step in MS treatment, which will hopefully allow many more patients crucial access to treatment.
About Synexa
At Synexa, we specialise in supporting clinical trial analysis and have a particular focus on biologic drugs like Natalizumab. By developing custom bioanalytical assays, we are able to support studies examining biosimilars including examining drug pharmacokinetics and immunogenicity. If you would like to learn more please reach out at contactus@synexagroup.com.
References:
- Kawamoto, Okamoto and Shimaoka. Autoimmune Dis. 2012;2012:357101
- Steinman L. No quiet surrender. J Clin Invest. April 1, 2015;125(4):1371–1378





