Cancer cachexia is a silent thief. It robs patients of strength, stripping away muscle and fat, leaving them frail and exhausted. For many living with advanced cancer, this wasting syndrome is not just a symptom – it’s a life-threatening condition that nutrition alone cannot reverse. Behind the scenes, a complex storm of inflammation and metabolic disruption drives this relentless decline.
Enter S-pindolol, a molecule with unique potential. This single enantiomer of racemic pindolol may offer a triple advantage in cancer cachexia: slowing catabolism through nonselective β-blockade, boosting anabolism via partial β₂ receptor agonism, and lifting appetite and energy through serotonin receptor activity. An early clinical trial hinted at something remarkable – patients not only stopped losing weight, they began regaining fat-free mass.
Bioanalysis
Bioanalysis is the foundation of evidence-based drug development ensuring that new therapies are both safe and effective before reaching patients. Validated bioanalytical methods are required for clinical trial data submission.
Alderley Analytical, now part of Synexa, were proud to be chosen to support a comparative Phase I bioavailability study of S-pindolol and racemic pindolol.
At Synexa’s Manchester LC-MS/MS facility, the team rose to the occasion. Our team developed a chiral chromatography method that could separate R- and S-pindolol with surgical accuracy. Using a Lux Cellulose-1 column and LC-MS/MS detection, they validated the assay to the highest regulatory standards.
Method Development and Validation
- The method was fully validated to regulatory guidelines over the calibration range 0.25 to 100 ng/ml for each enantiomer
- Excellent accuracy & precision were demonstrated
- Stability in plasma was established through 3 freeze/thaw cycles and in thawed samples for 24 hours at room temperature
- R– and S-pindolol were stable in whole blood for 1 hour at room temperature
- Matrix effects were investigated in 6 human plasma donors, including a haemolysed and lipemic sample
All other validation experiments passed the acceptance criteria.
Long-term stability (LTS) Testing in Human Plasma
- Two sets of QC samples were prepared and stored at -80 °C and -20 °C
- Acceptable storage stability was demonstrated at both temperatures over 67 days and 175 days
Phase 1 Bioequivalence Study
A total of 1,465 clinical samples were collected during the study period from December 2021 to June 2022. Study samples were stored for a maximum of 123 days. Samples were analysed for R– and S-pindolol using the validated chiral method in 31 analytical batches. QC performance was acceptable throughout the study.
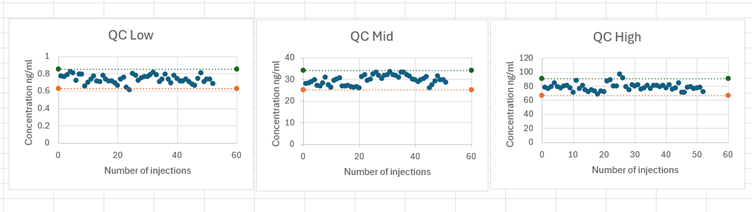
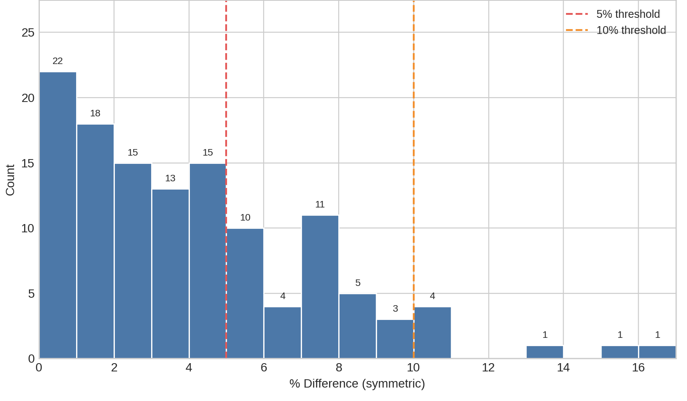
Incurred sample reanalysis (ISR) was performed on an ongoing basis. The ISR data demonstrated excellent performance of the method, with 100% of results being within the acceptance criteria of +/-20% difference from initial and repeat result. For the 123 samples analysed, 69% of results were within +/-5%.
Data from this bridging study of enantiomerically pure S-pindolol benzoate and its parent racemic drug, pindolol, support future clinical trials of S-pindolol benzoate for the treatment of cancer cachexia.
Following completion of the study we were delighted to receive this positive endorsement from Dr. Elaine Morten, Head of Regulatory Affairs and Technical Development at Actimed Therapeutics Ltd.
“Alderley Analytical provides a critical service to Actimed Therapeutics and has proven to be a first-class partner.
From obtaining cost estimates to the delivery of final reports, every element of our project is delivered professionally, promptly and to a high quality.
The working style is flexible and tailored, our working relationship is fully integrated, and communication is excellent. The team at Alderley is exemplary, responsive and knowledgeable. I would not work with any other vendor in this field, and I cannot recommend Alderley highly enough.”
To read more about the trial, you can access the publication here:
https://doi.org/10.1002/jcsm.13651
About Synexa Life Sciences
At Synexa, our UK site is equipped with state-of-the-art UPLC-MS/MS platforms, ideally positioned just 15 minutes from Manchester Airport to support rapid turnaround for clinical studies. Operating from a GLP/GCP-accredited facility, we provide seamless bioanalytical and biomarker support throughout the entire drug development continuum – from pre-clinical to clinical phases.
Beyond LC-MS/MS, our comprehensive analytical capabilities, including ligand binding and flow cytometry assays, enable in-depth insights into post-translational modifications and other complex biological processes.
If you would like to learn more about how we can support your bioanalysis or biomarker needs, visit www.synexagroup.com.
FAQ
How can biomarkers support development of therapies for cancer cachexia?
Cachexia involves metabolic, inflammatory, and muscle‑depletion processes that require detailed biological monitoring. Synexa’s proteomics, cytokine profiling, and immune‑functional assays identify pharmacodynamic signals that indicate therapeutic benefit. These insights clarify whether a therapy influences key pathways linked to muscle preservation and systemic inflammation. This enables developers to identify effective candidates earlier.
What makes cachexia difficult to study and how does Synexa address it?
Cachexia varies widely among patients, making consistent measurement challenging. Synexa integrates multi‑platform analyses—such as proteomics, immune profiling, and PK/PD data—to identify stable mechanistic markers. These markers help differentiate genuine treatment effects from disease‑driven variability. This supports clearer decision‑making during early clinical evaluation.
How can Synexa help differentiate anti‑cachexia therapies in a competitive landscape?
Differentiation depends on demonstrating unique activity across metabolic and inflammatory pathways. Synexa maps biomarker signatures and pathway interactions using multi‑modal platforms. These insights highlight distinctive mechanistic features that strengthen scientific positioning. This evidence supports regulatory justification and competitive advantage.





