Are antibody-drug conjugates (ADCs), principally comprised of an antibody, a cytotoxic payload and a linker, the next therapeutic modality of choice for oncology? A global market share of $7.82 billion in 2022, expected to grow annually by 11.2% from 2023 to 2030, and 4 FDA approvals in the last three years, suggests so. However, despite there being 11 FDA approvals since 2000 for at least 20 indications, this is not to say there aren’t any challenges.
In our latest blog, we delve into this therapeutic modality, the requirements for their bioanalysis, challenges in their development and the latest innovations in the field.
Considerations for ADC Bioanalysis
Measuring a drug’s pharmacokinetics (PK) is a key objective in any clinical study. ADCs are no different. However, their inherent composition makes PK assays particularly complex. Many ADCs have cleavable linkers, and the possibility of untethered payloads cannot be ignored. Therefore, assays need to be able to quantify:
- Total antibody
- Conjugated antibody
- Free payload
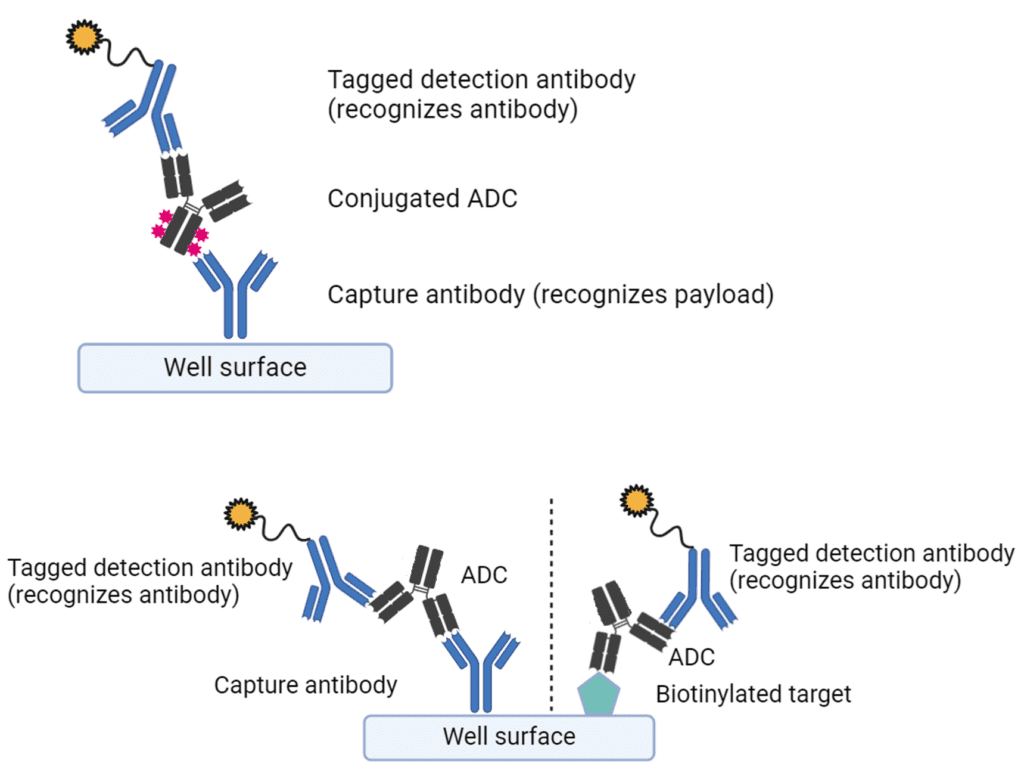
Figure 1. Assay builds for PK ADC assays
Assay builds use specific capture reagents against a combination of the antibody and conjugated payload components to differentially recognise conjugated and unconjugated forms of the ADC. Most often, the free payload is quantified by LC/MS/MS assays. The requirement for up to 3 separate PK assays can, in turn, raise the costs of ADC bioanalysis. The performance of these assays will also be highly dependent on the drug/antibody ratio, which is a key parameter during method validation.
Whilst many ADCs have an IgG1 backbone, which can limit immunogenicity, the presence of anti-drug antibodies needs to be addressed and detected in clinical studies. Like other biologics, ADC immunogenicity detection follows a tiered screening approach, followed by confirmation. What makes ADCs slightly different is that confirmation further confirms the domain specificity of the anti-drug antibody response. i.e., whether anti-drug antibodies specifically recognise the antibody, linker or payload components. Depending on their functional impact, neutralising anti-drug antibodies can be detected in either cell-based or ligand-binding assays. Clinically, not only can anti-drug antibodies inhibit ADC function, but antibodies against the payload and/or linker region can form complexes leading to off-target toxicity in immune cells.
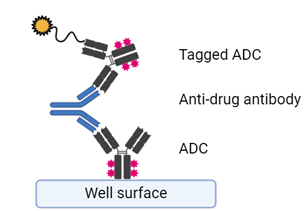
Figure 2. Basic build of screening anti-drug antibodies for an ADC
The toxicity problem
Immunogenicity is not the only reason for toxicity from ADCs. Unfortunately, this is one of the primary challenges and limitations of the therapy. Toxicity can occur via two primary mechanisms:
- Non-tumor target antigen expression (specific toxicity)
- Free payload toxicity (non-specific toxicity)
The expression of target antigens by non-tumour cells, even at low levels, can kill off-target cells. Toxicity screening is often addressed during preclinical animal studies and often necessitates non-human primate models. To overcome drug toxicity challenges, investigators emphasise understanding the abundance of a target antigen and tailoring the antibody for maximum specificity. In addition, highly innovative drug builds can mask the antibody from non-tumour antigens. These and other innovations will be expanded on later.
Non-specific toxicity results in the depletion of certain non-tumour cell types. Some of the most common are thrombocytopenia (loss of platelets) and neutropenia (loss of neutrophils). In the case of neutropenia, neutrophils cleave the linker region, freeing the cytotoxic payload, which kills them. It is important that the risk of off-target cell death be addressed as soon as possible and can be screened for preclinically.
In vitro experimentation has demonstrated how only differentiating neutrophils become affected by ADC toxicity. Therefore, the first step is establishing an immature neutrophil population to detect the potential for neutropenia-related toxicity. This is achieved through preferential isolation of CD34+ progenitor cells from peripheral blood, followed by up to 10 days of culture in media supplemented with specific cytokines, growth factors and stimulants. After that, treatment with ADCs for up to 6 days and flow cytometry staining of CD66b expression can determine neutrophil-specific depletion. Similarly, for thrombocytopenia, CD34+ progenitors are first isolated and then cultured in a highly defined culture media for ten days prior to ADC exposure. Once again, a flow cytometry panel, specifically staining for megakaryocyte markers (CD45 and CD41), can determine the loss of megakaryocytes, the cells responsible for platelet production.
The resistance problem
Whilst toxicity will always be a concern for drug developers, tumour resistance remains a prominent hurdle. ADC resistance implies changes in the target cancer biology, which reduces cancer cell death and diminishes clinical efficacy. Heterogenous cancers have developed several resistance mechanisms, including downregulation of target antigens, overexpression of drug transporters to increase efflux, changes in ADC trafficking pathways, poor payload processing, and alterations in key signalling pathways, summarised in Figure 3.
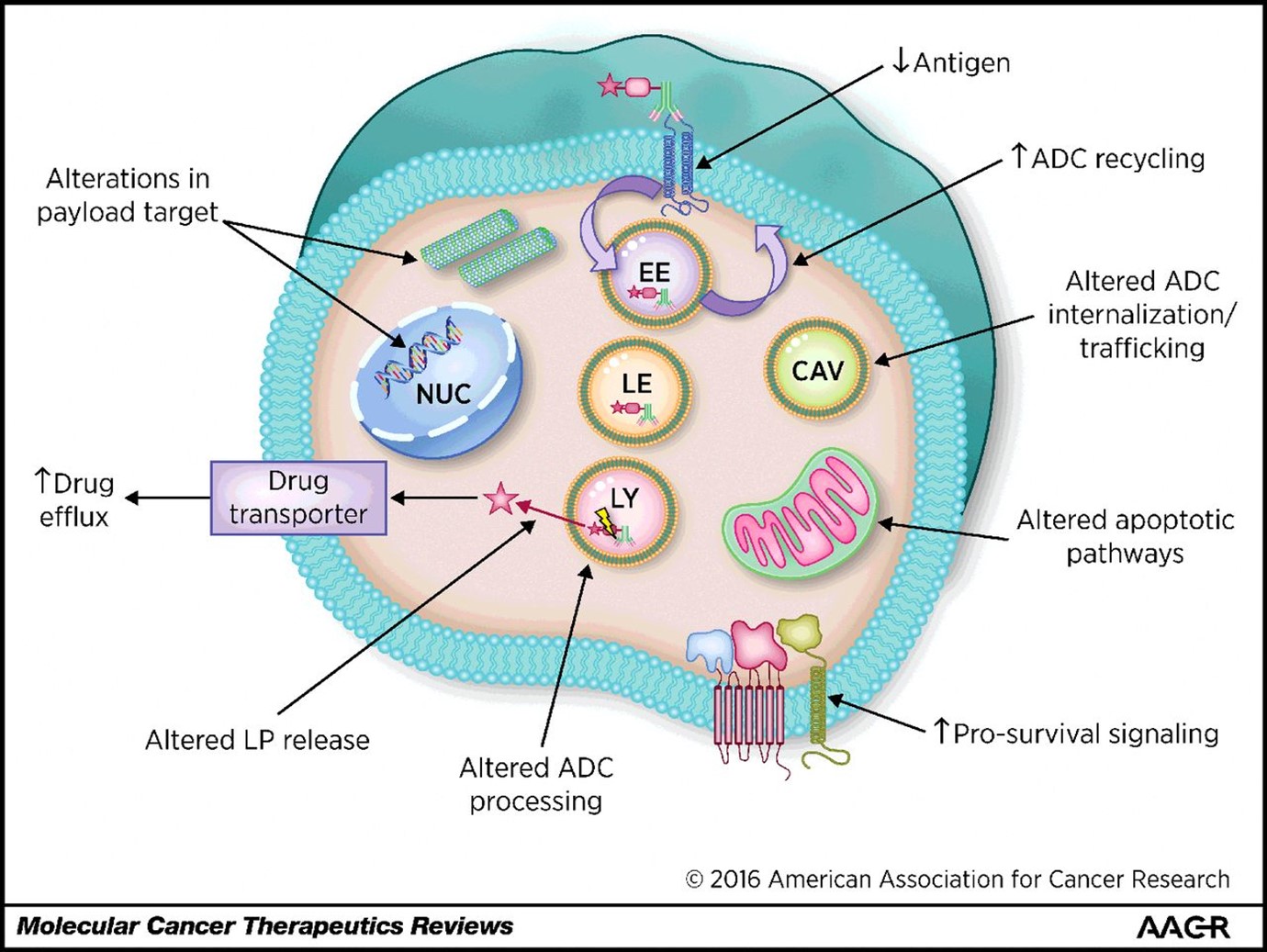
Figure 3. Common mechanisms of ADC resistance in cancer cells. From Loganzo, Sung, & Gerber. Mol Cancer Ther. 2016 Dec;15(12):2825-2834
It is essential, therefore, to predict potential resistance mechanisms in target cancers. Preclinically, this is accomplished with humanised xenograft animal models, transplanted with highly resistant tumours or using cell culture. The latter requires cyclical or sub-clinical dosing of ADCs to target cells to generate resistant lines. Resistance mechanisms can then be interrogated in greater detail, including antigen expression changes, genetic modifications to metabolic and transporter genes and imaging of lysosomal function and drug processing mechanisms. Overcoming both cancer resistance and reducing toxicity have bred highly innovative approaches in the latest generation of ADC therapies, summarised in our final section.
The new generation
Overcoming the ever-evasive target cancers necessitates a highly innovative approach. In the realm of ADCs, this can be distilled into three primary categories:
- Payload and linker design
- New target identification
- Effective biomarkers
Some of the issues regarding resistance are being addressed by changes to the fundamental design of ADCs, which in turn are hoped to lead to greater clinical efficacy. These include bispecific ADCs, which target multiple antigens. This approach may overcome particularly heterogenous cancers. Changes to the payloads may also address this via the bystander effect. By using more hydrophobic payloads, these can potentially be freed to target multiple adjacent cells, potentially those without the target antigen.
To address targeted toxicity wherein non-cancer cells express the target antigen, new-generation ADCs utilise probodies. These are antibodies which are conditionally activated. For example, the antigen binding site may be masked until the antibody is exposed to the highly acidic environment of the tumour microenvironment, thereby limiting binding to healthy cells.
Other novel designs to further improve drug efficacy include ISACs, immune-stimulating antibody conjugates, which combine antibodies and pattern recognition receptors like STING or TLRs. In this configuration, the ISACs recruit the innate immune system to attack the targeted cancer cells. Finally, dual-drug conjugates incorporate multiple distinct payloads to target resistant cancers better.
Final thoughts
In this blog, we have briefly touched on the basics of ADC build, their bioanalysis, and the challenges and opportunities that drug developers face in this space. What is abundantly clear is that this area is attracting significant investment and innovation to overcome fundamental challenges intrinsic to cancer. Addressing potential toxicity remains crucial, and preclinical evaluations can help predict non-cancer cell death before clinical application.
About Synexa
Synexa is a specialist biomarker and bioanalytical service lab supporting biopharma and biotech clients at each stage of drug development. We believe in collaborative and bespoke approaches to address our client’s needs. In the field of ADC drug development, we cannot only validate robust and appropriate assays to measure ADC PK and immunogenicity but also explore potential toxicities of ADC candidates using cell culture and multiparametric flow cytometry panels. If you would like to learn more, please contact us.
References
- Carrasco-Triguero et al. Bioanalysis. 2019 Sep;11(17):1555-1568.
- Khoury et al. Int J Mol Sci. 2023 Jun 2;24(11):9674.
- Loganzo, Sung, & Gerber. Mol Cancer Ther. 2016 Dec;15(12):2825-2834
- Naveh et al. J Transl Med. 2024 May 31;22(1):526.
- Raja, Kasana & Verma. Mol Biotechnol. 2024 Sep 2.
FAQ
Which PK and immunogenicity assays are essential for ADCs, and how do they interlock?
ADCs often require three PK analytes, total antibody, conjugated antibody, and free payload, paired with ADA and potentially NAb assessments. Synexa deploys ligand‑binding assays for antibody species, LC‑MS/MS for payload quantification, and tiered ADA workflows that map domain specificity across antibody/linker/payload. This interlocked design reveals exposure, deconjugation, and immune impact concurrently. Sponsors gain a coherent view of efficacy, safety, and manufacturability.
How do you anticipate and evaluate off‑target or bystander toxicity?
Synexa uses ex vivo bone‑marrow progenitor systems and immune‑phenotyping to probe haematologic risk, plus in vitro models to study bystander effects and lysosomal processing. Proteomic toxicity markers and cytokine panels help detect subclinical injury patterns. Where needed, payload‑specific immunogenicity panels assess antibody formation against linker/payload regions. These datasets inform linker chemistry choices, dosing, and monitoring windows.
What makes DAR‑aware method development so important?
Drug‑to‑antibody ratio influences sensitivity, dynamic range, and signal attribution across PK and ADA assays. Synexa designs DAR‑aware calibrators and capture strategies to avoid under‑ or over‑reporting conjugated species. LC‑MS/MS methods are tuned for payload release kinetics, and ligand‑binding formats are stress‑tested against likely DAR distributions. This ensures reliable, phase‑appropriate analytics from discovery through registration.