The recent announcement by the WHO last month to declare a public health emergency over recent outbreaks of mpox across multiple countries has sent a chill down health authorities’ spines. Following Covid-19, the world is on high alert for anything that could indicate another pandemic. What do we know about the recent outbreaks and how are we collectively working to prevent another worldwide pandemic?
History of mpox and mpox variants
Mpox was first identified as a distinct illness in 1958 among laboratory monkeys in Copenhagen, Denmark. The first documented human cases occurred in 1970, involving six unvaccinated children during the smallpox eradication efforts, with the first being a 9-month-old boy in the Democratic Republic of Congo (DRC). Since then, many more mpox cases have been reported in Central and West Africa. Originally thought to be uncommon in humans, cases have increased since the 1980s, possibly due to waning immunity following the cessation of routine smallpox vaccination.
Mpox is classified into two primary genetic groups, Clade I (previously referred to as the Central African or Congo Basin Clade) and Clade II (formerly known as the West African Clade). Clade I is further divided into Clade Ia and Clade Ib, while Clade II is divided into Clade IIb and Clade IIa, with smaller subgroup clusters known as lineages. Clade I, which is endemic to Central Africa, is associated with more severe illness and higher mortality rates, with some outbreaks resulting in up to 10% of those infected dying, although recent outbreaks have had lower death rates. In contrast, Clade II, endemic to West Africa, generally causes less severe infections, with more than 99.9% of people surviving.
Since May 2022, human mpox cases have been reported in multiple countries that previously had no known presence of the virus in animal or human populations. The global mpox outbreak that began in 2022 was primarily caused by Clade II, specifically lineage B.1 of Clade IIb. Things have taken a turn in the recent outbreak. In the DRC, Clade I, which has long spilled over from animal reservoirs to people was sometimes followed by limited spread from human to human. These cases were concentrated in the west and centre of the country, mainly affecting children. Recently, Clade I, specifically Clade Ib, has now also spread to Uganda, Kenya, Rwanda, and Burundi, triggering worries about further spread as travellers from Africa have carried the virus to Sweden and Thailand. However, Clade II mpox has also continued to circulate, with more than 1,000 cases detected in the U.S. alone in the first six months of 2024.
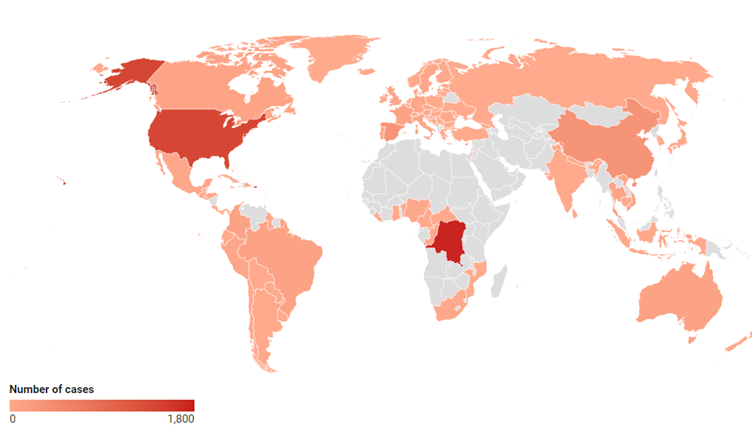
The map below shows where mpox has been reported since the start of 2024, according to data from the World Health Organisation.
Transmission
The recent surge in mpox cases can be attributed to several interrelated factors that have emerged over time. A key reason is a decline in population immunity following the eradication of smallpox in 1980 and the discontinuation of the smallpox vaccine, which also offered protection against mpox. This has left a growing number of people, particularly those born after the end of smallpox vaccination, vulnerable to the mpox virus. Additionally, spillovers from animals to humans may have become more frequent due to the near-extinction of large forest animals, pushing people to hunt smaller rodents that are more likely to carry the virus. The expansion of farming deeper into forests has also brought humans into closer contact with these infected animals. Furthermore, factors such as population growth, urbanisation, and increased human mobility have amplified the chances of the virus spreading from person to person.
The Clade Ib virus has drawn particular attention because it appears to transmit more easily between people than earlier strains, including through sexual activity, while Clade Ia primarily spreads from animals to humans. Genetic analyses suggest that Clade Ib has been circulating in humans for some time, as its genome contains mutations likely induced by the human immune system, in contrast to Clade Ia, which has fewer of these mutations. However, experts caution that these mutations and Clade distinctions may not be the most critical factors in understanding the spread of mpox. The region where the virus circulates, and the local population dynamics could play a more significant role. For instance, Clade Ia is more common in sparsely populated rural areas, limiting its spread, whereas Clade Ib is emerging in densely populated regions, where it spreads more readily. Despite these insights, much remains unknown about mpox transmission. Scientists have yet to identify the primary animal reservoir for the virus in the wild, though rodents are suspected carriers. This uncertainty highlights the complexity of the situation and the need for ongoing research to fully understand the factors driving the current outbreak.
Mpox vaccines, the boom in stock prices and ongoing clinical trials
The recent mpox outbreak has catalysed a surge in interest and investment in vaccines, significantly impacting the stock prices of vaccine manufacturers. The primary vaccines being utilised to combat mpox are JYNNEOS, developed by Bavarian Nordic, and LC16m8, produced by KM Biologics. JYNNEOS, which contains a non-replicating poxvirus, and LC16m8, which features a live but weakened poxvirus strain, were originally created for smallpox but are proving crucial in addressing mpox. As the outbreak intensified in 2022, Bavarian Nordic’s share price experienced a substantial boost, reflecting increased demand and optimism about revenue prospects. The company’s stock saw considerable gains as global attention focused on the need for effective mpox vaccines. In contrast, KM Biologics, with its LC16m8 vaccine primarily used in Japan, has seen more modest fluctuations in its share price. Despite this, the broader vaccine market has been energised by the crisis, with increased investments and interest in vaccine production and distribution. The ongoing response to mpox, including efforts to expand vaccine access and conduct further research, continues to drive stock market activity, demonstrating the significant economic impact of global health emergencies on vaccine manufacturers.
Clinical trials have primarily evaluated these vaccines against Clade II, which has been the predominant strain in recent outbreaks in Europe and the U.S. In these contexts, JYNNEOS has shown an effectiveness of about 80% with one dose and 82% with two doses in preventing mpox. However, this data mostly comes from trials involving young, healthy men who have sex with men, a group particularly affected during the 2022 outbreak. The response of vaccines against Clade I and its subtypes, especially in children and those with weakened immune systems, is less well understood. Research is ongoing to fill these gaps. A forthcoming clinical trial in the DRC aims to assess JYNNEOS in individuals who have had close contact with mpox but are asymptomatic, to determine its effectiveness in preventing or mitigating the disease.
Despite the promise of these vaccines, availability remains a critical issue. While several wealthy nations have pledged to donate doses to Africa, including the U.S. and the European Union, delivery has been delayed. The World Health Organisation has yet to approve the vaccines for use in low- and middle-income countries, complicating distribution plans. Experts, including those in the DRC, estimate that up to 10 million doses are needed to effectively address the outbreak, but current supplies fall short. The uncertainty around vaccine arrival and distribution has hindered efforts to form effective vaccination strategies, particularly in vulnerable populations such as children and sex workers. This situation highlights the challenges of managing a global health crisis and the need for swift, coordinated action to prevent further spread of mpox.
About Synexa
Synexa Life Sciences offers a comprehensive array of solutions designed to assess the efficacy and immunogenicity of vaccine candidates. Our vaccine services are meticulously designed to investigate both cellular and humoral immune responses, providing a holistic view of vaccine efficacy. Utilising advanced techniques such as flow cytometry, MHC multimer analysis and cutting-edge immunoassays, we enable customers to delve deeply into the immune responses elicited by vaccines. Through rigorous evaluations, we empower customers with the critical data needed to make informed decisions, facilitating the development of vaccines that are both safe and effective.
References
Americo, J. L., Earl, P. L. & Moss, B. Proc. Natl Acad. Sci. USA 120, e2220415120 (2023).
Yinka-Ogunleye, A. et al. BMJ Glob. Health 8, e013126 (2023).
Yeganeh, N. et al. Vaccine 42, 125987 (2024).
Priyamvada, L. et al. Vaccine 40, 7321–7327 (2022).
Monzón, S. et al. Nature Commun. 15, 3059 (2024).
Vakaniaki, E. H. et al. Preprint: medRxiv https://doi.org/10.1101/2024.04.12.24305195 (2024).
https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
https://www.niaid.nih.gov/diseases-conditions/mpox-vaccines
FAQ
How should vaccine studies evolve given clade‑specific differences in mpox epidemiology?
Assay strategies should capture clade‑relevant antigenicity and functional neutralisation while keeping platforms comparable across clades I/II lineages. Synexa supports multiplex serology, cellular immunity (ELISpot/FluoroSpot), and controlled neutralisation formats, aligning readouts to evolving public‑health guidance. By harmonising panels, cross‑clade immunogenicity can be interpreted without confounding assay effects. This enables data‑driven updates to strain selection and booster policy.
What bioanalytical signals are most predictive of protection or severe‑disease risk?
Neutralising activity is important but insufficient alone; coordinated T‑cell responses and innate signatures influence protection and disease course. Synexa correlates humoral titres with T‑cell functionality and soluble inflammatory markers to build composite correlates. Longitudinal sampling maps durability and identifies waning windows for booster timing. This multidimensional approach strengthens evidence for label expansions and public‑health recommendations.
How can sponsors prepare for changing outbreak dynamics while studies are ongoing?
Prospective contingency planning is crucial: define adaptive endpoints, interim analyses, and bridging strategies to accommodate new clades or transmission shifts. Synexa maintains assay lineage and cross‑panel comparability so emergent variants can be incorporated without resetting programs. Rapid method updates are managed under controlled change processes to preserve validity. This keeps development on track even as epidemiology evolves.





