Glioblastoma remains one of the most aggressive and treatment-resistant cancers. Standard therapies often fail, leaving patients with limited options. In a study published in Nature Medicine in March 2024, researchers explored the use of IL-13Rα2-targeted chimeric antigen receptor (CAR) T cells delivered directly to the tumour site in patients with recurrent high-grade gliomas (rHGG), including recurrent glioblastoma (rGBM). This Phase 1 trial provides exciting insights into the bioactivity of CAR-T cells demonstrating a multi-faceted approach to measure treatment response and efficacy.
The study examined locoregional delivery of CAR-T cells through intratumoural (ICT), intraventricular (ICV), or combined ICT/ICV routes in 65 patients with rHGG. The primary focus was on safety, feasibility, and biological markers of efficacy, providing a comprehensive look at how CAR-T cells behaved in the brain tumour environment. Among the tools used to assess the CAR-T cells’ effectiveness were cytokine profiling, flow cytometry, PCR, and immunohistochemistry (IHC).
Key findings include:
- Locoregional delivery was feasible and well-tolerated
- No dose-limiting toxicities were identified
- Arm 5 (dual ICT/ICV with optimised manufacturing) had the best overall survival (OS) of 10.2 months
- CAR-T cells triggered cytokine responses, and increased IFNγ-pathway activation correlated with better patient outcomes
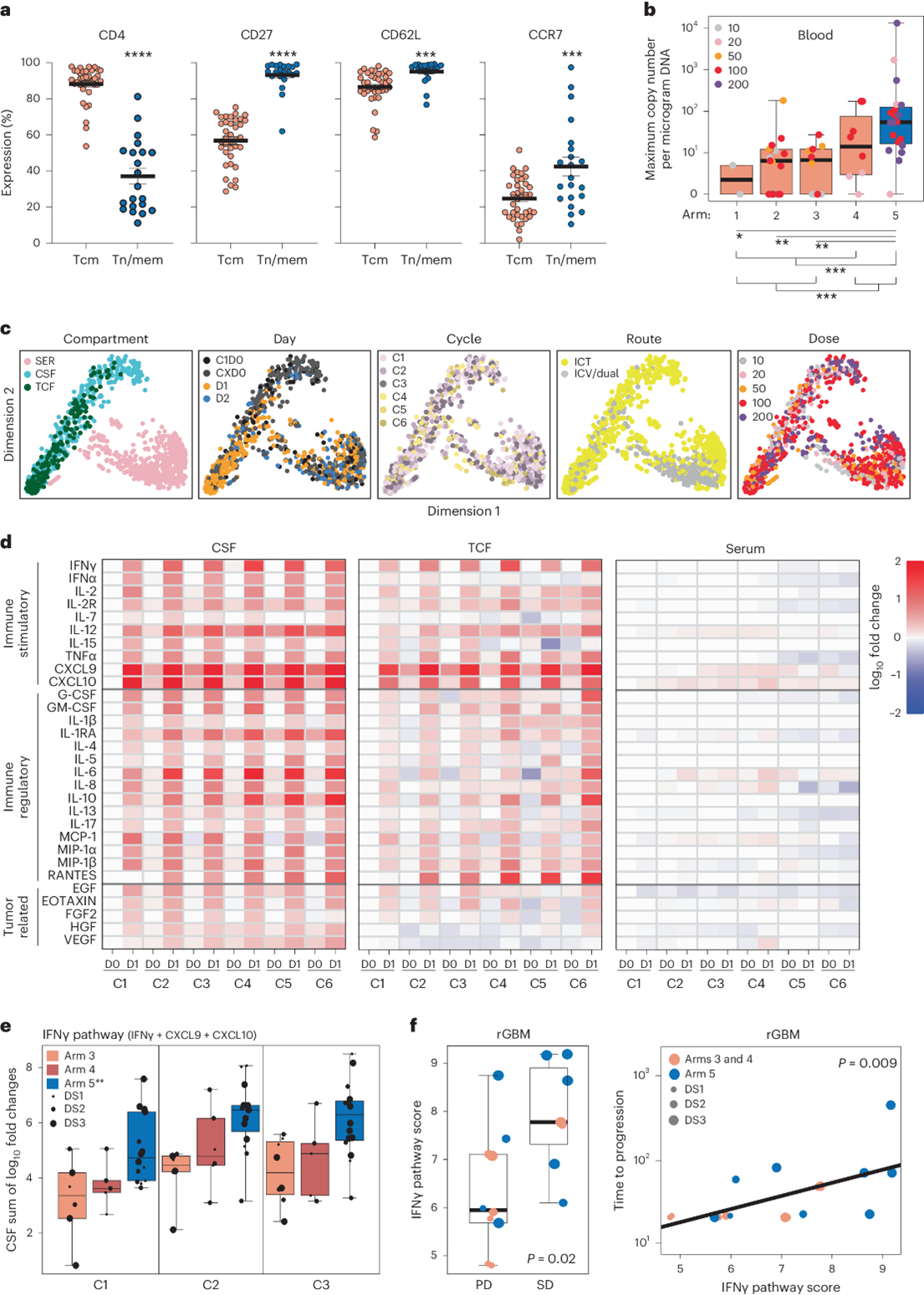
Fig 1: a Expression of CD4, CD27, CD62L, and CCR7 on Tcm- and Tn/mem-derived CAR+ T cells, shown with means and standard error. Significant differences indicated by P-values (****P < 0.0001; ***P ≤ 0.0005). b WPRE copy number per microgram of PBMC DNA shown in a box-and-whisker plot, with significant differences between treatment arms (P ≤ 0.0003).c Cytokine measurements represented by low-dimensional plots, coloured by sample compartment (CSF, TCF, or serum), treatment day, and dose. d Heatmap of cytokine levels across cycles and days (pre- and post-infusion), showing log10 fold changes from baseline in CSF, TCF, and serum. e IFNγ pathway score changes shown in a box-and-whisker plot for patients in arms 3, 4, and 5, with significant differences (P ≤ 0.0006) between arms. f CSF IFNγ pathway score plotted against response and time to progression, highlighting correlation with better outcomes. Significant differences indicated by P-values. Adapted from: Brown, C.E., Hibbard, J.C., Alizadeh, D. et al. Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: a phase 1 trial. Nat Med 30, 1001–1012 (2024). https://doi.org/10.1038/s41591-024-02875-1
Cytokine Biomarkers: Tracking Immune Activation
The study highlights the significant impact of cytokines on the clinical outcomes. Following CAR-T cell administration, significant spikes in inflammatory cytokines were observed in the cerebrospinal fluid (CSF) and tumour cavity fluid (TCF), particularly those associated with the IFNγ pathway, including IFNγ, CXCL9, and CXCL10. These elevations persisted across treatment cycles, suggesting that CAR-T cells elicited a sustained immune response within the central nervous system (CNS). The activation of the IFNγ pathway played a central role in this immune response, with increased levels of IFNγ and its downstream cytokines strongly correlated with improved clinical outcomes. Notably, patients in Arm 5, who received dual ICT/ ICV CAR-T cell delivery, exhibited the highest IFNγ-pathway activation, and this group achieved the longest overall survival (OS) of 10.2 months, indicating a direct link between cytokine dynamics and survival.
Other immune-stimulatory cytokines, including IL-12 and IL-2R, showed slight increases post-infusion, further supporting the immune-enhancing environment created by the CAR-T cells. Concurrently, decreases in tumour-promoting cytokines such as HGF and VEGF were observed in the TCF, indicating that the therapy may also suppress factors contributing to tumour growth.
Flow Cytometry: Defining CAR-T Cell Phenotypes
By leveraging flow cytometry, the study was able to pinpoint which T cell subsets were more likely to persist in the body and mount a durable anti-tumour response. This is crucial because the persistence and fitness of CAR-T cells directly influence their ability to eliminate tumours.
The trial utilised two distinct manufacturing platforms: Tcm-derived CAR-T cells (central memory T cells) and Tn/mem-derived CAR-T cells (naïve and memory T cells). Flow cytometry revealed that Tn/mem-derived CAR-T products had a more favourable immune phenotype, with a higher proportion of CD27+, CD62L+, and CCR7+ cells, markers associated with longer-lasting immune memory and reduced T cell exhaustion.
Flow cytometry also highlighted differences in CD4+ and CD8+ T cell subsets, providing insights into the balance between helper and cytotoxic T cells in the CAR-T products. A balanced CD4/CD8 ratio was identified as another positive indicator of therapeutic potential.
qPCR: Monitoring CAR-T cell persistence
Quantitative PCR (qPCR) played a crucial role in evaluating the persistence of CAR-T cells in the CSF, TSF and blood. CAR-T cells were detectable in the CSF and TCF for several days post-infusion, with a notable subset of patients demonstrating CAR-T cell persistence beyond 7 days. This persistence in the CSF is particularly significant given that the CSF typically undergoes rapid turnover, cycling approximately four times per day. The ability of CAR-T cells to remain in such a dynamic environment for extended periods underscores their sustained activity and potential efficacy in targeting tumour cells.
CAR-T cell detection in the blood was most strongly associated with the dual ICT/ICV delivery method used in Arm 5, the treatment arm that achieved the best overall survival outcomes of 10.2 months. This suggests that dual delivery not only enhances CAR-T cell distribution within the CNS but also facilitates systemic circulation of CAR-T cells, potentially contributing to more robust and widespread anti-tumour activity.
These qPCR findings emphasise the importance of CAR-T cell persistence and trafficking, particularly the ability of cells to migrate from the CNS into the peripheral blood, which could have implications for broader tumour targeting and therapeutic success.
Immunohistochemistry (IHC): Visualising T Cell Infiltration and Tumour Microenvironment
IHC was used to assess intratumoural immune cell infiltration, specifically looking at CD3+ T cells in the tumour microenvironment before treatment. This provided critical insights into the relationship between tumour immune contexture and patient outcomes. The study revealed that patients with intermediate to high CD3+ T cell infiltration in their tumours prior to CAR-T treatment experienced significantly better survival outcomes. These patients, characterised by a “hot” tumour microenvironment, had a median overall survival (OS) of 11.2 months, compared to only 6.5 months for those with low CD3 infiltration. Notably, two patients with the highest CD3 T cell scores achieved a complete response (CR), indicating that the pre-existing immune presence in the tumour plays a critical role in enhancing CAR-T cell efficacy. CD8+ cytotoxic T cells were also more prevalent than FOXP3+ regulatory T cells (Tregs) in tumours with higher CD3 scores, further suggesting that an immune-active environment is critical for CAR-T therapy success.
Bioanalytical integration: the importance of multimodal assessment
This study highlights the importance of using multiple bioanalytical techniques to gain a comprehensive understanding of a CAR-T therapy’s biological effects. The integration of these bioanalytical tools not only provided a deeper understanding of how CAR-T cells function in glioblastoma but also identified biomarkers that can guide future treatment strategies.
At Synexa Life Sciences, we offer the expertise needed to fully assess the performance of novel CAR-T therapies in complex and challenging environments. From cytokine profiling to deep immunophenotyping, we deliver the critical data required to optimise your CAR-T cell products, ensuring they achieve maximum efficacy and provide personalised patient care.
For further details, the full study can be accessed here:
https://www.nature.com/articles/s41591-024-02875-1#Sec11
FAQ
How do you structure bioanalysis when CAR‑T cells are delivered locoregionally in GBM?
Compartmental sampling (CSF, tumour cavity fluid, and serum) is vital to connect local pharmacology with systemic signals. Synexa aligns compartment‑specific cytokine profiling, flow cytometry for persistence/exhaustion markers, and q/dPCR for CAR transgene with paired safety labs. Temporal mapping reveals compartmental kinetics and IFN‑pathway activation that may associate with response. This framework informs dose routes, schedules, and endpoint selection.
What readouts best predict durable activity in brain tumours?
Converging evidence, from CSF cytokine signatures, CAR‑T phenotypic persistence, and intracranial IFN‑related pathway scores, can flag durable immune pressure. Synexa integrates these with radiographic and clinical endpoints to build composite response biomarkers. Assay lineage from translational work to clinic allows consistent interpretation across phases. These composites support responder identification and adaptive trial design.
How do you mitigate neuro‑inflammatory risk while maintaining efficacy?
Define escalation rules around intracranial cytokine kinetics and T‑cell activation markers, not just systemic CRS signals. Synexa’s rapid‑turnaround panels help teams pre‑empt neurotoxicity by adjusting dose density or route. Proteomic markers of endothelial activation can further warn of blood–brain barrier stress. This risk‑managed approach keeps potency without compromising patient safety.