During the COVID-19 pandemic, safe administration of the vaccine was sequentially tested in younger and younger patient groups. The FDA has approved the safe use of the current COVID vaccines in children 6 months and older. Whilst safety is of paramount concern, vaccine efficacy is also crucial in all patient groups. In adult populations, venous blood sampling for the purposes of quantifying antibody titres against the vaccine antigen is easy to accomplish. These assays examine the humoral immune response (of different antibody subtypes, including IgM, IgG and IgA) to the viral protein the vaccine is based on. In the case of COVID-19, this was (in most vaccines), a portion of or the complete spike protein. The difficulty in studies which include children is the requirement of sampling. It is useful in efficacy studies to understand a time course of immune response with multiple sampling time points, often with volumes of at least 5 mL of venous blood drawn. This is of course, not possible in pediatric patients.
A solution to this is patient-centric microsampling. In the case of humoral response, various microsampling devices permit the collection of dried blood spots (DBS). These include Trajan’s hemaPEN®, the Capitainer®, Neotyrex Mitra® and Tasso Devices. While each device is different, the central principle involves drawing of a droplet of blood using a lancet and immobilization on a membrane. The DBS sample can then be eluted in a buffer and evaluated in analytical assays.
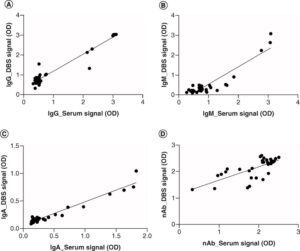
Synexa, in collaboration with Trajan, published an article in 2021, which demonstrated validation of a high-throughput ELISA assay for the detection of IgM, IgG, IgA and neutralizing antibodies against the SARS-CoV-2 spike protein. Importantly, part of the assay development was a comparison between a traditional phlebotomy matrix, serum, and a DBS matrix. Results indicated good correlations between serum and DBS samples taken from the same individual (Maritz et al, 2021 and shown below). These individuals had prior SARS-CoV-2 infection with humoral antibody levels similar to vaccinated individuals (unpublished data). This data is just one such example of how DBS samples may be used to monitor response to vaccination. Importantly, DBS allows the evaluation of pediatric patients, requiring a minimally invasive sample collection.
DBS sampling, therefore, serves both as an effective means of monitoring immune response in immunized pediatrics and also for remote sampling which is particularly useful during a pandemic where public congregation (at testing facilities for example) may perpetuate disease spread.
See our published article available here: https://www.future-science.com/doi/full/10.4155/bio-2021-0065?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
Ref:
Maritz L, Woudberg NJ, Bennett AC, Soares A, Lapierre F, Devine J, Kimberg M, Bouic PJ. Validation of high-throughput, semiquantitative solid-phase SARS coronavirus-2 serology assays in serum and dried blood spot matrices. Bioanalysis. 2021 Aug;13(15):1183-1193. doi: 10.4155/bio-2021-0065. Epub 2021 Jun 11. PMID: 34114884; PMCID: PMC8202508.