One of the main challenges translational researchers face is the search for the ultimate preclinical model, a model that not only perfectly captures the disease in question with nuance and accuracy, but also allows high throughput testing and accurate assessment of drug response. Arguably, there cannot be a more difficult model to capture than that of the brain, high in sheer complexity and functional integration. Couple that with trying to integrate disease modelling, for example, understanding brain tumour formation, and the challenge becomes even greater. On World Brain Tumour Day, we highlight some of the exciting translational tools that have been developed to understand brain cancer and how these are allowing a closer transition into the clinic for paediatric brain cancer treatment.
Paediatric brain tumours are the most common type of solid childhood cancer, second only to leukaemia as a cause of paediatric malignancies. In recent years, research efforts have improved our understanding of the underlying molecular landscape of paediatric brain cancers, contributing to clinical advances and improving patient life expectancy. Despite this, current treatments are limited and require risk-adapted treatment protocols to improve cure rates using new therapeutic regimens. Molecular intertumoural and intratumoural heterogeneity are among the main factors contributing to the failure of numerous clinical studies, highlighting the need for appropriate models that can capture physiological responses to specific treatments. Enter patient-derived (PDOs) organoids, a powerful new platform to use at the forefront of personalized medicine.
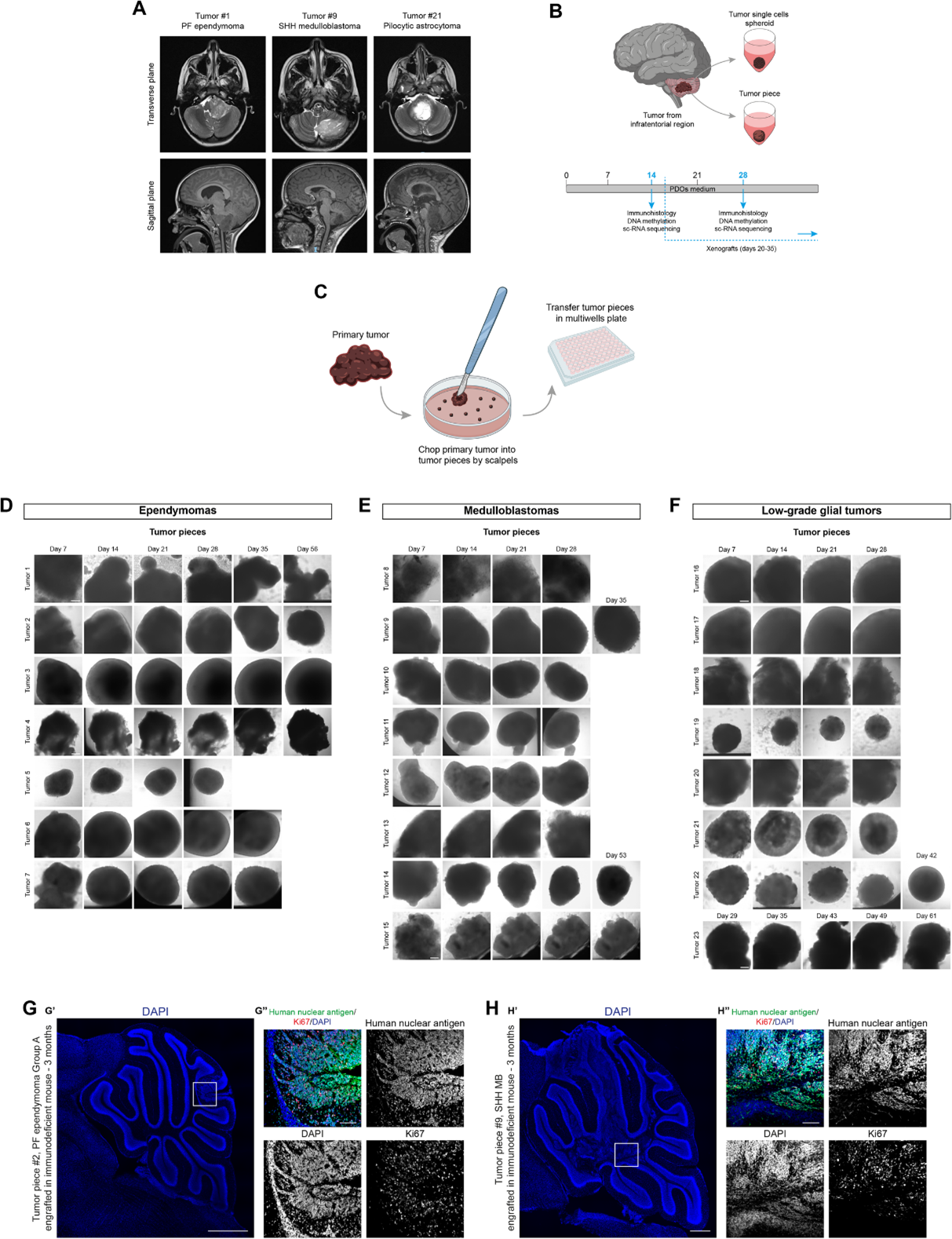
In a paper published in EMBO Molecular Medicine, the authors describe the successful generation and characterisation of PDOs from paediatric brain tumour biopsies and patient‐derived xenografts. Through comprehensive analysis, they were able to demonstrate that PDOs maintain tumour histological characteristics, DNA methylation and mutational profiles. They also maintained the tumoural heterogeneity and the cellular morphology and architecture of the primary tumours, as shown by scRNA sequencing and immunohistological analysis. PDOs and PDXOs could be largely amplified and biobanked, maintaining remarkable fidelity to their parental tumours, even after many passages in culture.
From a personalised medicine standpoint, the PDOs were highly sensitive in recapitulating patient-specific responses. PDOs derived from two specific tumour types (ependymoma and medulloblastoma) responded similarly to established treatment protocols when compared to the original patients. Interestingly, treated PDOs failed to engraft in mice, potentially mirroring the patient’s disease remission. Furthermore, PDOs derived from different patients within the same tumour type showed some variation in response, suggesting the model’s potential to capture individual patient responses.
While brain organoid models for paediatric brain cancer research are still in their early stages, these studies showcase the immense promise they hold for the future. With continued advancements and as the technology matures, these models can be further refined to integrate with microfluidic technologies and immune cell co-cultures to create even more robust models for personalized medicine approaches, ultimately offering more effective and targeted therapies for children battling this devastating disease.
References
Lago, C., Federico, A., Leva, G., Mack, N. L., Schwalm, B., Ballabio, C., & Tiberi, L. (2023). Patient‐ and xenograft‐derived organoids recapitulate pediatric brain tumour features and patient treatments. EMBO Molecular Medicine, 15(12). https://doi.org/10.15252/emmm.202318199
Subramanian S, Ahmad T. Childhood Brain Tumors. Stat Pearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK535415/
Abdullah KG, Bird CE, Buehler JD, Gattie LC, Savani MR, Sternisha AC, Xiao Y, Levitt MM, Hicks WH, Li W et al (2022) Establishment of patient‐derived organoid models of lower‐grade glioma. Neuro Oncol 24: 612–623. https://doi.org/10.1093/neuonc/noab273
Adolph JE, Fleischhack G, Mikasch R, Zeller J, Warmuth‐Metz M, Bison B, Mynarek M, Rutkowski S, Schüller U, von Hoff K et al (2021) Local and systemic therapy of recurrent ependymoma in children and adolescents: short‐ and long‐term results of the E‐HIT‐REZ 2005 study. Neuro Oncol 23: 1012–1023. https://doi.org/10.1093/neuonc/noaa276





