Custom Solutions
Services / Custom Solutions
Custom Solutions & Novel Modalities
Bespoke Solutions that Elucidate Novel Therapies & Modalities
Synexa was founded to support the global biopharmaceutical industry with bespoke end-to-end bioanalytical solutions in order to effectively achieve their clinical milestones, supporting the development of a wide range of therapeutics from discovery through phase IV.
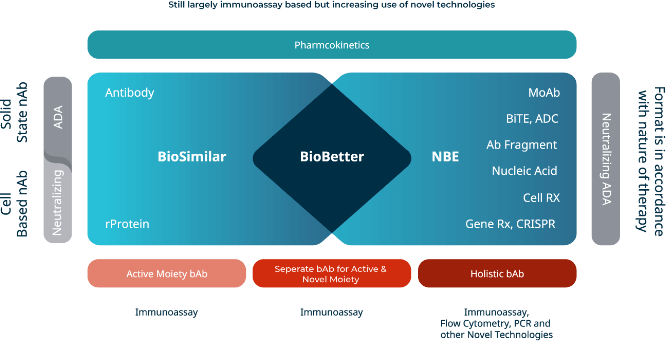
Our experience extends into the field of novel modalities and bespoke assay development as needed for each study. We strive to identify, implement and integrate the optimum analytical methodologies for Biosimilars, BioBetters and all other therapeutic drug pipelines.
Services
Overview
Custom Assay Development
Thorough assay development, optimisation and validation is best done well in advance of planned studies as the process can deliver significant improvements in assay sensitivity and overall robustness, with implications for sample volume and sample preparation methods. We conduct this process collaboratively with you as the client in order to ensure the assay meets your required needs.
- Method Development
The purpose of method development is to define and test the design of a method under a variety of conditions, before subjecting it to validation with basic assay parameters being optimized prior to method validation. Such parameters include but are not limited to the assay platform, suitable reference standard, quality controls, matrix type, minimum required dilution, assay quantification range, sample collection processes, storage conditions, incubation temperatures and duration, capture and detection molecule pairs and concentrations and number of wash cycles.
- Validation
Bioanalytical method validation illustrates that an optimised method is suitable for the intended analysis of study samples. Synexa performs method validation according to the FDA and EMA guidelines. The extent of method validation (custom or full validation) is agreed upon with you and is outlined in a validation phase plan to suit the needs of the study.
Novel Modalities
Advances in new biologics gives hope for better treatment of complex diseases. At Synexa, we have vast experience in the development of bespoke assays for novel, as yet untested, large molecule modalities, developing and validating assays as per your needs.
In particular, we are pioneering the field of Exosomes/EVs analysis ,using microparticle-detecting flow cytometry, and are integrally involved in developing bioanalytical strategies for checkpoint inhibitor development.
- Exosomes
Flow cytometric microparticles analysis is being developed for analysis of microparticles in CSF for stratification of neurological diseases such as Parkinson’s Disease. Growing evidence indicates that microparticles are prominent mediators of neurodegenerative diseases, thus making these an attractive therapeutic modality to be explored.
- Immune Checkpoint Inhibitors
The development of immune checkpoint inhibitors (ICIs) targeting lymphoid cells has remarkedly transformed therapeutic approaches for the treatment of cancer. Synexa has established flow cytometry panels to characterise circulating immune cell subsets and determine the effects on ICI therapies.
Innovative Therapies
As new therapies are developed, it is necessary for innovative technologies and bioanalytical strategies to be utilised to evaluate and assess each therapeutic and it’s potential.
Synexa is at the forefront of developing and implementing these strategies in key therapeutic areas and niche treatment approaches.
- Cell & Gene Therapies
Our team of scientists have a wide range of experience in the area of Cell & Gene therapies and can provide integral guidance to your study, giving comprehensive guidance in selection or development of relevant assays.
- Vaccines
Our experience in the underpinning immunobiology of health and disease give us the foundation to provide expert guidance supporting vaccine development across a wide array of therapeutic areas, including COVID-19.
- Biosimilars
Biosimilars are a fast-growing sector within the pharmaceutical industry. Our experience in this field makes us well-suited to provide high-quality, relevant and informative bioanalytical expertise to your therapeutic’s development.
We use innovative, industry-proven and trusted technology platforms to ensure we provide only best data for your purpose.
Platform
Meso Scale Discovery
Platform
ELISA
Platform
Flow Cytometry
Platform
NanoString
Platform